Marc Laboratories Ltd. is privately held pharmaceutical companies in India, headquartered at Lucknow, in the state of Uttar Pradesh.
Over the last three decades, it has been developing and manufacturing pharmaceutical products and selling and distributing these in India. An integrated healthcare solutions provider with pharmaceutical product basket, it caters to a wide range of therapeutic areas that include orthopaedic, gyaenaecology, cardiovascular, gastrointestinal, analgesics, haematinics, anti-infectives, antibiotics, and antidiabetics and immunologicals. The company focuses on providing high quality, appropriately priced products to its customers. Marc Laboratories has a multilingual workforce of more than thousand employees.
The company has Manufacturing establishment in Baddi, Himanchal Pradesh and Gurgaon, Haryana.
The company has state-of-the-art manufacturing facilities conforming to the most stringent international WHO-GMP norms. Spread over Two Lac sq.ft. of land, Marc Laboratories' manufacturing facility at Baddi, Himanchal Pradesh is the cynosure of all eyes, well equipped with world-class production facilities.
A responsible corporate conscious of its duty towards various sections of the society; Marc Laboratories nurtures young talents and believes in bringing talent to it's best.
- Company Name:Marc Laboratories Ltd.
(View Trends)
-
Health, Wellness and Fitness
-
500 - 1,000 employees
- 9484835 Global Rank
- 488785 India
-
Search100.00%
-
Direct0.00%
-
Display0.00%
-
Mail0.00%
-
Referrals0.00%
-
Social0.00%

- India 93.4%
- Otomotiv
- Üreticiler ve Distribütörler
- 1 K Downloads
- 10 SDKs
- 3.62 Avg. Rating
- 13 Total reviews

- App Url: https://play.google.com/store/apps/details?id=com.patientportalpp
- App Support: http://marclabs.in
- Genre: medical
- Bundle ID: com.patientportalpp
- App Size: 41.1 M
- Release Date: September 5th, 2019
- Update Date: September 5th, 2019
Description:
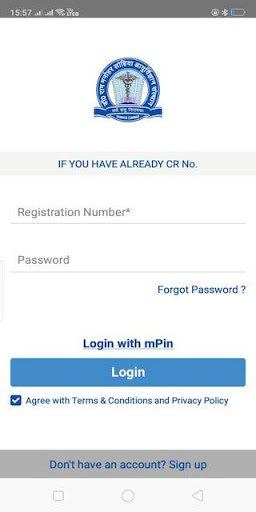
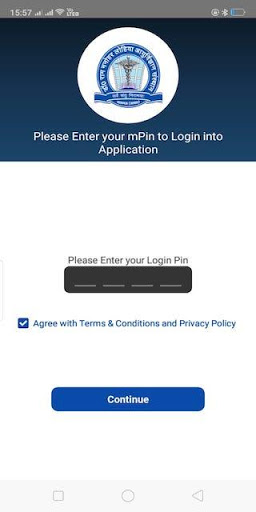
This App Provides Patients of DRRMLISM Lucknow the factility to book appointsment , see his reports



 Learnium
Learnium
 Oblador
Oblador





