MylanÍs unconventional beginning traces back to 1961, when company co-founders, Milan ñMikeî Puskar and Don Panoz, traveled to small towns in rural West Virginia distributing medicine to those in need. Since then, weÍve remained focused on providing access to high quality medicine and setting new standards in healthcare.
MylanÍs mission _ to reach the worldÍs 7 billion people _ is an ambitious one, and itÍs why weÍve built a workforce of more than 35,000 people who are passionate about their work. People who are curious and entrepreneurial. People who understand that, when it comes to helping those in need, thereÍs no time to wait.
Our team works in offices and manufacturing facilities around the world, including global centers in Bangalore, India; Canonsburg, Pennsylvania (U.S.); and Dublin, Ireland. MylanÍs global research and development (R&D) centers are located in Hyderabad, India, and Morgantown, West Virginia (U.S.), and we have 12 technology-focused R&D sites across Europe, India, Japan and the U.S. At MylanÍs ~50 manufacturing facilities worldwide, we adhere to the highest quality standards.
Mylan has a growing portfolio of more than 7,500 marketed products that serve patients in more than 165 countries and territories. Already, more than 40% of people worldwide being treated for HIV/AIDS with an antiretroviral depend on a Mylan product.
In addition to our commitments to patients, customers, shareholders and communities, Mylan is committed to the environment and workplace safety. With our global environmental, health and safety program, Mylan is focused on investing in processes and technologies to reduce our environmental footprint.
Count on Mylan to keep striving to deliver better health for a better world, one person at a time. Learn more at Mylan.com.
- Company Name:Mylan
(View Trends)
-
Headquarters: (View Map)Hatfield, United Kingdom
-
Pharmaceuticals
-
> 10,000 employees
- 1390385 Global Rank
- 982031 India
- 73 K Estimated Visits
-
Search41.31%
-
Direct40.82%
-
Referrals17.15%
-
Social0.72%
-
Display0.00%
-
Mail0.00%
-
29.65%
-
19.40%
-
7.55%
-
3.79%
-
3.06%

- United States 54.8%
- India 4.1%
- Romania 2.6%
- Biotechnology and Pharmaceuticals
- Pharmaceuticals
- 10 SDKs
- 0 Total reviews

- App Url: https://itunes.apple.com/app/mylan/id1006876526
- App Support: http://mylan.com
- Genre: Medical
- Bundle ID: com.mylan.ultiva.ap
- App Size: 21.3 M
- Version: 1.5.4
- Release Date: June 26th, 2015
- Update Date: August 9th, 2017
Description:
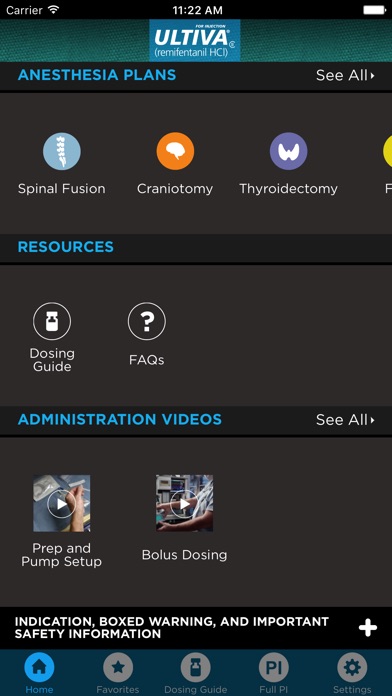
Put Remi* anesthesia plans at your fingertips. This app gives you easy access to plans featuring ULTIVA® (remifentanil HCl) for Injection CII.
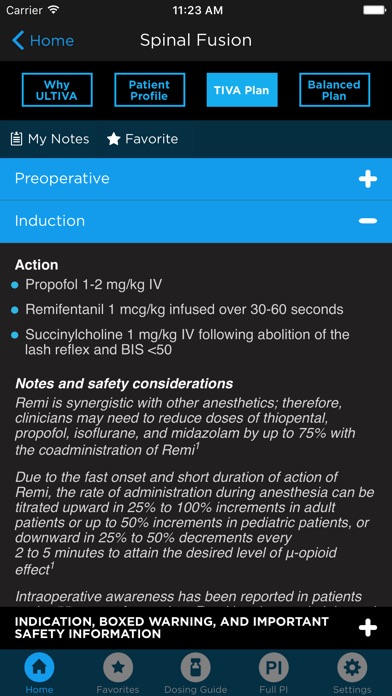
• Learn from anesthesia plans for spine, orthopedic, ENT, cardiac, and more.
• Expand your knowledge with a variety of TIVA and balanced anesthesia plans.
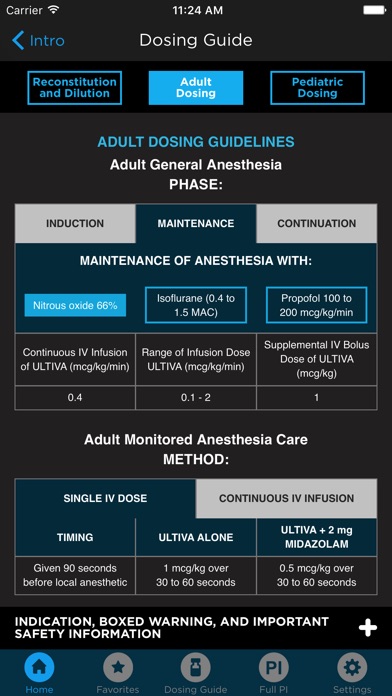
• Quickly review Remi dosing guidelines with easy access to a 4-page guide.
THIS APP IS INTENDED ONLY FOR ANESTHESIA PROVIDERS IN THE UNITED STATES. USE OF ANY DRUG, THERAPEUTIC REGIMEN, OR MEDICAL TECHNIQUE MENTIONED IN THIS APP IS SUBJECT TO THE INDEPENDENT KNOWLEDGE AND JUDGMENT OF THE PRACTITIONER.
INDICATION AND IMPORTANT SAFETY INFORMATION, INCLUDING BOXED WARNING
Indication
ULTIVA is indicated for IV administration:
• As an analgesic agent for use during the induction and maintenance of general anesthesia for inpatient and outpatient procedures.
• For continuation as an analgesic into the immediate postoperative period in adult patients under the direct supervision of an anesthesia practitioner in a postoperative anesthesia care unit or intensive care setting.
• As an analgesic component of monitored anesthesia care in adult patients.
Important Safety Information
WARNING: ADDICTION, ABUSE, AND MISUSE
ULTIVA exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient’s risk prior to prescribing ULTIVA.
Please visit https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dbc63b6e-f8c5-4fd0-8ec3-4f5e19125313 to see the remainder of the Important Safety Information and download the ULTIVA full Prescribing Information, including boxed warning.
If you have questions or comments, please call us at 800.RX.MYLAN.
ULTIVA is a registered trademark of Glaxo Group Limited. The Mylan logo is a registered trademark of Mylan Inc.
*Remifentanil is commonly referred to as Remi by anesthesia providers.

 AVFoundation
AVFoundation
 CFNetwork
CFNetwork
 Core Foundation Framework
Core Foundation Framework
 Core Graphics
Core Graphics
 Core Location Framework
Core Location Framework
 Foundation Framework
Foundation Framework
 Quartz Core Framework
Quartz Core Framework
 Security Framework
Security Framework
 System Configuration F...
System Configuration F...
 UIKit
UIKit
-
Native0.00%
-
Standard50.00%
-
Direct50.00%






They are headquartered at Hatfield, United Kingdom, and have 23 advertising & marketing contacts listed on Kochava. Mylan works with Advertising technology companies such as DoubleClick.Net, Google Floodlight Counter, JW Player Tracking.





