Vericel develops, manufactures, and markets autologous cell-based therapies for patients with serious diseases and conditions. The company markets three cell therapy products in the United States. MACI¬ (autologous cultured chondrocytes on porcine collagen membrane) is a third generation autologous cellularized scaffold product that is indicated for the repair of single or multiple symptomatic, full-thickness cartilage defects of the adult knee, with or without bone involvement. Carticel¬ (autologous cultured chondrocytes) is an autologous chondrocyte implant intended to treat cartilage defects in the knee in patients who have had an inadequate response to a prior arthroscopic or other surgical repair procedure. Epicel¬ (cultured epidermal autografts) is a permanent skin replacement for the treatment of patients with deep dermal or full thickness burns greater than or equal to 30% of total body surface area. Vericel is also developing 1 additional cell product. Ixmyelocel-T is a multicellular therapy intended to treat advanced heart failure due to ischemic dilated cardiomyopathy (DCM).
Developing autologous (patientÍs own) cell therapies„with integrity
Vericel uses rigorous scientific methods to develop novel therapies for the treatment of patients with autologous (patientÍs own) cells. In addition, personal integrity, team work, collaboration, and innovative technology are the foundations of our work. We seek to practice transparency in our clinical trials and research, and in our relationships with each other, our patients, and the investors who support us.
- Company Name:Vericel Corporation
(View Trends)
-
Headquarters: (View Map)Cambridge, MA, United States
-
Biotechnology
-
200 - 500 employees
- 2035728 Global Rank
- 406069 United States
-
Direct59.19%
-
Search23.81%
-
Social6.63%
-
Referrals5.59%
-
Mail4.78%
-
Display0.00%

- Housing Associations
- 50 Downloads
- 10 SDKs
- 0 Total reviews

- App Url: https://play.google.com/store/apps/details?id=com.vericel.mymacirehab
- App Support: https://www.maci.com/patients/
- Genre: health_and_fitness
- Bundle ID: com.vericel.mymacirehab
- App Size: 31.2 M
- Release Date: February 8th, 2021
- Update Date: February 8th, 2021
Description:
Ready to make your comeback with the MACI knee cartilage repair procedure?
It’s your move.
Common side effects include joint pain, tendonitis, back pain, joint swelling, and joint effusion.
More serious side effects include joint pain, cartilage or meniscus injury, treatment failure, and osteoarthritis.
Please see Full Prescribing Information at MACI.com for more information. https://www.maci.com/pdf/Final-MACI-PI-FINAL.pdf
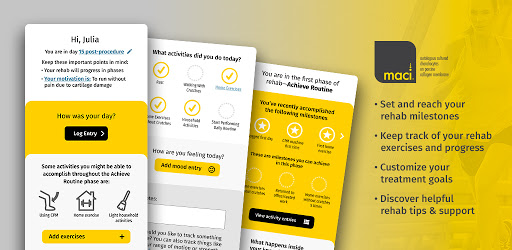
Use the My MACI App to personalize your recovery experience and help you stay motivated throughout your rehab journey.
• Prepare for your MACI surgery and plan for your recovery
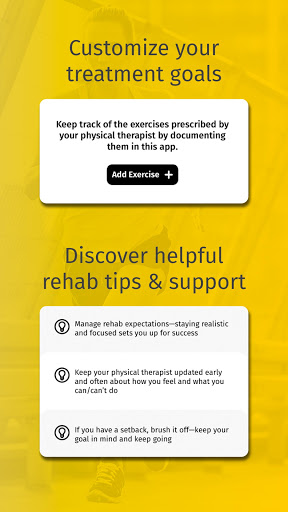
• Customize your treatment goals
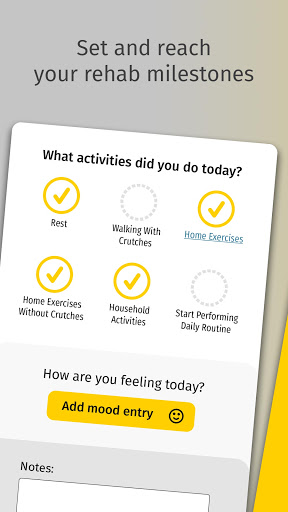
• Set and reach milestones
• Keep track of your rehab exercises and progress
• Discover helpful rehab tips & support from real patients
Indication and Important Safety Information
INDICATION
MACI® (autologous cultured chondrocytes on porcine collagen membrane) is made up of your own (autologous) cells that are expanded and placed onto a film that is implanted into the area of the cartilage damage and absorbed back into your own tissue.
MACI is used for the repair of symptomatic cartilage damage of the adult knee.
The amount of MACI applied depends on the size of the cartilage damage. The MACI film is trimmed by your surgeon to match the size and shape of the damage, to ensure the damaged area is completely covered.
Limitations of Use
It is not known whether MACI is effective in joints other than the knee.
It is not known whether MACI is safe or effective in patients over the age of 55 years.
IMPORTANT SAFETY INFORMATION
MACI should not be used if you:
• are allergic to antibiotics such as gentamicin, or materials that come from cow, pig, or ox;
• have severe osteoarthritis of the knee, other severe inflammatory conditions, infections or inflammation in the bone joint and other surrounding tissue, or blood clotting conditions;
• have had knee surgery in the past 6 months, not including surgery for obtaining a cartilage biopsy or a surgical procedure to prepare your knee for a MACI implant;
• or cannot follow a doctor-prescribed rehabilitation program after your surgery
Consult your doctor if you have cancer in the area of the cartilage biopsy or implant as the safety of MACI is not known in those cases.
Conditions that existed before your surgery, including meniscus tears, joint or ligament instability, or alignment problems should be evaluated and treated before or at the same time as the MACI implant.
MACI is not recommended if you are pregnant.
MACI has not been studied in patients younger than 18 or over 55 years of age.
Common side effects include joint pain, tendonitis, back pain, joint swelling, and joint effusion.
More serious side effects include joint pain, cartilage or meniscus injury, treatment failure, and osteoarthritis.
Please see Full Prescribing Information at MACI.com. https://www.maci.com/pdf/Final-MACI-PI-FINAL.pdf
PP.US.MAC.1179 v2.0