AliveCor, Inc. is pioneering the creation of FDA-cleared 'machine learning' techniques to enable proactive heart care and is recognized around the world for transforming cardiac care. The FDA-cleared Kardia Mobile is the most clinically validated mobile ECG solution on the market and is recommended by leading cardiologists and used by people worldwide for accurate ECG recordings. This simple-to-use mobile device and app-based service provides instant analysis for detecting atrial fibrillation (AF) and normal sinus rhythm in an ECG. Kardia Pro is the first AI-enabled platform for doctors to monitor patients for the early detection of atrial fibrillation, the most common cardiac arrhythmia that leads to a five times greater risk of stroke. AliveCor was recognized by Fast Company as one of 2017's most innovative companies in health (#3). AliveCor is a privately-held company headquartered in Mountain View, Calif. For more information, please visit alivecor.com.
- Company Name:Alivecor Inc.
(View Trends)
-
Headquarters: (View Map)San Francisco, CA, United States
-
Medical Devices
-
10 - 50 employees
- 474316 Global Rank
- 181505 United States
- 336 K Estimated Visits
-
Direct53.58%
-
Search28.22%
-
Referrals17.33%
-
Display0.84%
-
Mail0.03%
-
Social0.00%
-
58.08%
-
10.93%
-
4.89%
-
3.43%

- United States 82.4%
- India 5.4%
- Children, Youth and Family
- Advocacy Groups
- 10 SDKs
- 4.73 Avg. Rating
- 999 Total reviews
- App Url: https://itunes.apple.com/app/alivecor-inc-/id579769143
- App Support: http://www.alivecor.com/support/
- Genre: Medical
- Bundle ID: com.alivecor.professional.aliveecg
- App Size: 685 M
- Version: 5.17.1
- Release Date: January 6th, 2013
- Update Date: June 15th, 2021
Description:
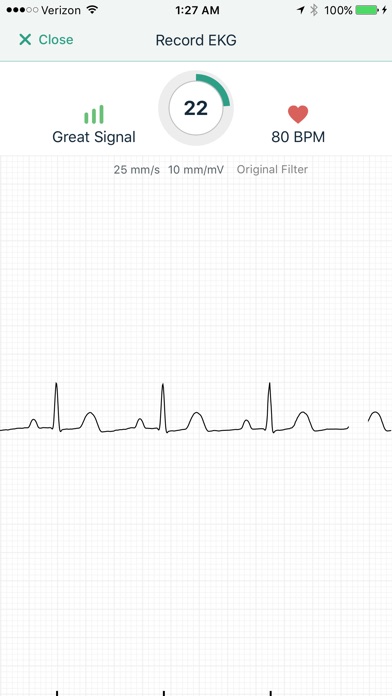
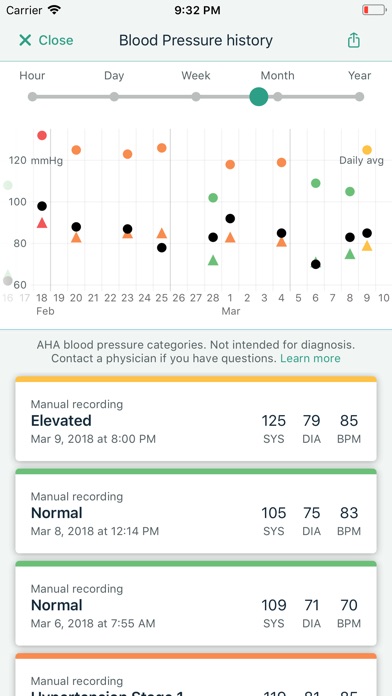
Kardia works with the FDA-cleared KardiaMobile, KardiaMobile 6L, or KardiaBand personal EKG devices, which can detect the most common arrhythmias in just 30 seconds. The Kardia app is designed to make managing heart care from home easier than ever, giving you the ability to seamlessly record EKGs, share heart data with your doctor remotely, keep track of your health history, and more.
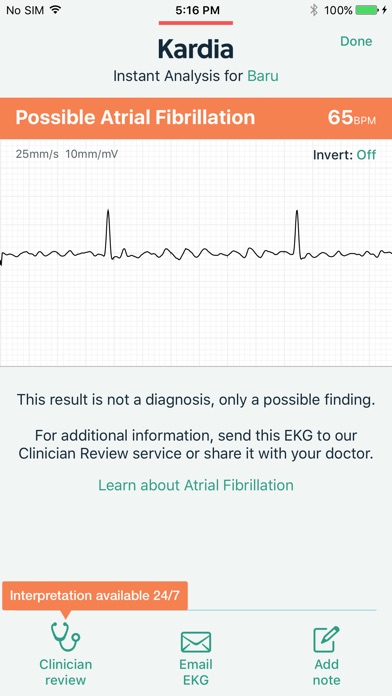
Capture a medical-grade EKG with your Kardia device anytime, anywhere—no patches, wires, or gels required. Get an immediate result from Kardia's Instant Analysis of normal, possible atrial fibrillation, bradycardia, or tachycardia. For additional analysis, you can choose to send the recording to your physician or to one of our partners for a Clinician Review by a cardiologist (US, Australia only) or cardiac care physiologist (UK, Ireland only).
The Kardia system is recommended by leading cardiologists and used by people around the world for accurate EKG recordings. Track your heart health data from home with the medical accuracy your doctor can trust.
NOTE: This app requires KardiaMobile, KardiaMobile 6L, or KardiaBand hardware to record an EKG. Get your Kardia device now at alivecor.com.
Kardia integrates with Apple Health to create a combined view of your health metrics.
NOTE: Requires KardiaMobile or KardiaBand hardware to record an EKG
FDA 510(k) numbers: K191406, K181823, K171816, K183319
CE # 0123

Sort by
davidbailey.uk
AZWarrior
Note to Developer
robert124589
Requires a subscription!!!
⚡️🫀
Great device, but-
CNDLG
Still charged for cancelled trial
Casher02
Scam
-
Standard80.00%
-
Direct20.00%


















They are headquartered at San Francisco, CA, United States, and have advertising & marketing contacts listed on Kochava. Alivecor Inc. works with Advertising technology companies such as Google Adsense, DoubleClick.Net, LiveRail, AppNexus, Neustar AdAdvisor, Openads/OpenX, Yahoo Small Business, X Plus One, Pubmatic, Rubicon Project, Aggregate Knowledge, Rocket Fuel, SpotXchange, Index Exchange, Tapad, Advertising.com, DemDex, Adap.TV, IponWeb BidSwitch, Geniee, BrightRoll, eXelate, AdRoll, Facebook Custom Audiences, Magnetic, NetSeer, Twitter Ads, Ad Tech Japan AOL, Criteo OneTag, Criteo, Amazon Ad System, Amazon Associates, Google Remarketing, Google Floodlight Counter, Bizo, LinkedIn Ads, DoubleClick Bid Manager, Podsights, Outbrain, Taboola, The Trade Desk, Outbrain Pixel.






Kardia 6L