For nearly 50 years, ECRI Institute, a nonprofit organization, has been dedicated to bringing the discipline of applied scientific research to discover which medical procedures, devices, drugs, and processes are best, all to enable you to improve patient care. As pioneers in this science, we pride ourselves in having the unique ability to marry practical experience and uncompromising independence with the thoroughness and objectivity of evidence-based research.
We are designated an Evidence-Based Practice Center by the U.S. Agency for Healthcare Research and Quality and listed as a federal Patient Safety Organization by the U.S. Department of Health and Human Services.
To explore career opportunities at ECRI Institute in Plymouth Meeting, Pennsylvania, please go to www.ecri.org and click on Careers on the Home page.
- Company Name:Ecri Institute
(View Trends)
-
Headquarters: (View Map)Plymouth Meeting, PA, United States
-
Hospital & Health Care
-
200 - 500 employees
- 381194 Global Rank
- 82011 United States
- 73.1 K Estimated Visits
-
Search59.99%
-
Direct32.66%
-
Referrals6.96%
-
Social0.29%
-
Mail0.10%
-
Display0.00%
-
73.55%
-
5.68%
-
2.41%
-
1.71%
- United States 16.8%
- Philippines 13.3%
- Public Health and Safety
- Policy and Regulation
- 10 SDKs
- 4.36 Avg. Rating

- App Url: https://itunes.apple.com/app/ecri-institute-1/id1438526754
- App Support: https://www.ecri.org/contact/
- Genre: Business
- Bundle ID: org.ecri.alertstracker
- App Size: 21.2 M
- Version: 1.2.2
- Release Date: March 14th, 2019
- Update Date: May 11th, 2020
Description:
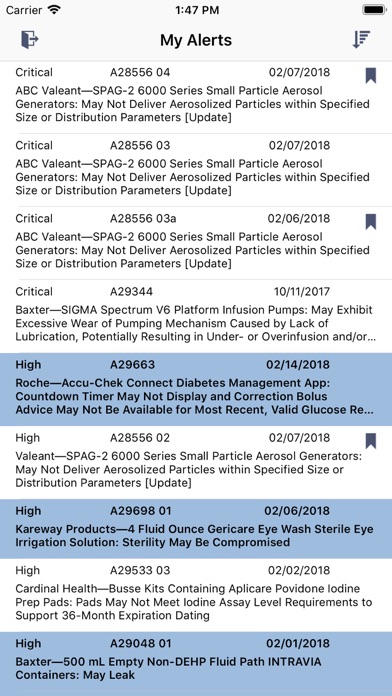
Disclaimer: This app is part of ECRI Institute’s Alerts Tracker® solution for product safety and alert/recall management. It is only available to current Alerts Tracker members.
ECRI Institute’s Alerts Tracker is easy-to-use recall management software that delivers daily alert notifications, tracks recalls from assignment to completion, and generates reports to ensure the removal of harmful products before they reach the patient. With a user-friendly design, the Alerts Tracker mobile app makes the recall management process even faster, easier, and more efficient. Manage new alerts for medical devices, blood products, food products, and pharmaceuticals away from your desk. Identify and remove products that could be harmful to patients. Document steps taken while at your inventory. Actions taken in app are immediately updated in the desktop version of Alerts Tracker, meaning you’ll be ready for the next Joint Commission survey or FDA regulatory audit.

-
Native0.00%
-
Standard93.75%
-
Direct6.25%








They are headquartered at Plymouth Meeting, PA, United States, and have 2 advertising & marketing contacts listed on Kochava. Ecri Institute works with Advertising technology companies such as DoubleClick.Net, Google Remarketing, DoubleClick Bid Manager, The Trade Desk.